Navigation auf uzh.ch
Navigation auf uzh.ch
Femtosecond Vibrational Spectroscopy
Focus of our research is to establish novel spectroscopic methods in the infrared (IR) and far-IR (THz) spectral range, which resolve the dynamics of structures of molecular systems and the energy flow through them on a very fast femtosecond timescale. IR spectroscopy analyzes nuclear motions of molecular systems and hence directly those degrees of freedom, which are relevant for their conformation. A large variety of questions is addressed, ranging from complex problems such as allostery, protein folding, energy transport in biomolecules, to fundamental structural processes in liquids like water.

2D-IRSpectroscopy
2D-IR spectroscopy, first demonstrated by Peter Hamm [1], developed into a powerful spectroscopic tool by now to investigate structure and dynamics of solution phase systems with unprecedented detail [2]. 2D-IR spectroscopy measures local contacts in a way similar to 2D-NMR, albeit with many orders of magnitudes higher time-resolution. It thus combines essentially unlimited time resolution with significant structure resolution. We have also introduced the method of transient 2D-IR spectroscopy, which extends conventional 2D-IR spectroscopy to the non-equilibrium regime, allowing us to make 'molecular movies' of fast conformational changes [3].
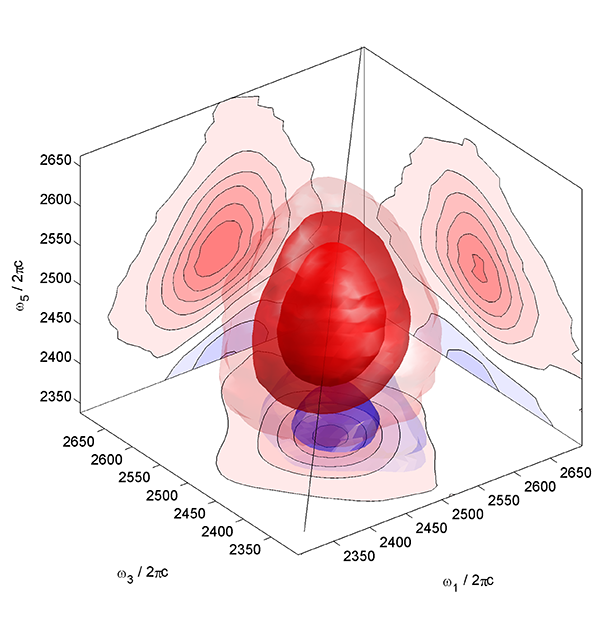
We are constantly pushing the development of new spectroscopic methods. For instance, we have been developing 3D-IR spectroscopy, both experimentally and theoretically, which allows one to resolve in much more detail the non-trivial stochastic processes of, e.g., the complicated hydrogen-bond network of liquid water [4]. More recently, we concentrate on 2D-Raman-THz spectroscopy, which measures the low-frequency intermolecular degrees of freedom of a liquid more directly, and reveals the structural inhomogeneity of the complex hydrogen bond networks of water [5-7].
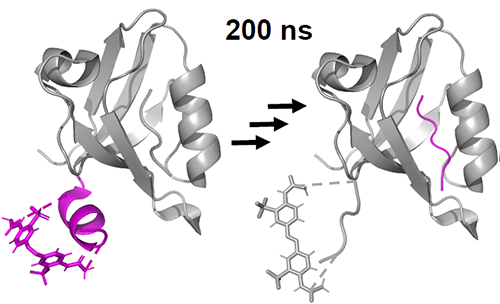
The ultimate goal of transient IR and 2D-IR spectroscopy is to be able to investigate in unprecedented detail complicated structural processes as they occur, for example, during protein (or peptide) folding. To this end, we are developing various approaches to trigger such structural processes on ultrafast timescales, utilizing molecular switches that are covalently linked to a peptide or a protein [8]. We are then probing the response of the peptide by means of IR and 2D-IR spectroscopy, covering all relevant timescales from femtoseconds to milliseconds [9, 10]. We recently started to use this concept to elucidate the nature of the allosteric signal in allosteric proteins [11, 12].
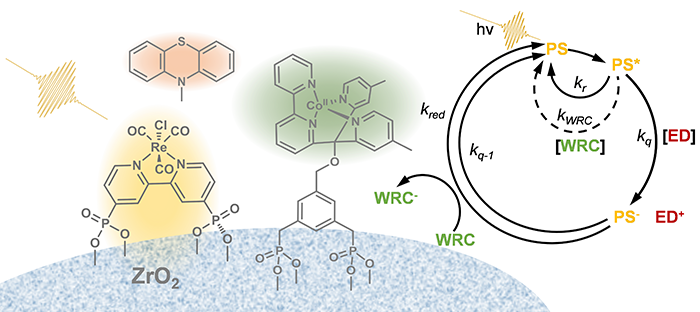
Molecular sciences very likely will become central in tackling one of the most pressing problems of humankind, i.e., the supply of renewable energies via some form of artificial photosynthesis. Artificial photosynthetic systems are perfectly suited to be investigated with transient IR spectroscopy, as they are intrinsically photo-triggerable. For example, we elucidated the electron transfer pathways between the various molecular components in a homogeneous hydrogen evolution system [13]. More recently, we also introduced surface sensitive IR spectroscopic techniques [14-16], as any future artificial photosynthetic system will most likely work with catalytic surfaces rather than in homogeneous solutions. CO2 reduction with molecular and solid state catalyst is an upcoming activity.
[1] P. Hamm, M. Lim, R. M. Hochstrasser, The Structure of the Amide I Band of Peptides Measured by Femtosecond Nonlinear IR Spectroscopy J. Phys. Chem. B 1998, 102, 6123-6138
[2] P. Hamm and M. T. Zanni, Concepts and Methods of 2D Infrared Spectroscopy, Cambridge, 2011
[3] C. Kolano, J. Helbing, M. Kozinski, W. Sander, P. Hamm, Watching Hydrogen-Bond Dynamics in a b-Turn by Transient 2D-IR Spectroscopy, Nature 444, (2006), 469
[4] S. Garrett-Roe, F. Perakis, F. Rao and P. Hamm, 3D IR Spectroscopy of Isotope Substituted Liquid Water Reveals Heterogeneous Dynamics, J. Phys. Chem. B, 2011, 115, 6976-6984
[5] J. Savolainen, S. Ahmed and P. Hamm, 2D Raman-THz Spectroscopy of Water, Proc. Natl. Acad. Sci. USA, 2013, 110, 20402-20407
[6] A. Shalit, S. Ahmed, J. Savolainen, and P. Hamm, THz Echoes Reveal the Inhomogeneity of Water in Aqueous Salt Solutions, Nature Chem., 2017, 9, 273-278
[7] A. Berger, G. Ciardi, P. Hamm and A. Shalit, The Impact of Nuclear Quantum Effects on the Structural Inhomogeneity of Liquid Water, Proc. Natl. Acad. Sci. USA, 2019, 116, 2458–2463
[8] J. A. Ihalainen, B. Paoli, S. Muff, E. H. G. Backus, J. Bredenbeck, G. A. Woolley, A. Caflisch, P. Hamm, α-Helix Folding in the Presence of Structural Constraints, Proc. Natl. Acad. Sci. USA 2008, 105, 9588
[9] B. Jankovic, J. Ruf, C. Zanobini, O. Bozovic, D. Buhrke, and P. Hamm, Sequence of Events During Peptide Unbinding from RNase S: A Complete Experimental Description, J. Phys. Chem. Lett. 2021, 21, 5201-5207
[10] P. Hamm, Transient 2D IR Spectroscopy from Micro- to Milliseconds, J. Chem. Phys., 2021, 154, 104201
[11] O. Bozovic, B. Jankovic and P. Hamm, Sensing the Allosteric Force, Nat. Commun., 2020, 11, 5841
[12] O. Bozovic, J. Ruf, C. Zanobini, B. Jankovic, D. Buhrke, P. J. M. Johnson, and P. Hamm, The Speed of Allosteric Signaling Within a Single-Domain Protein, J. Phys. Chem. Lett., 2021, 12, 4262-4267
[13] A. Rodenberg, M. Orazietti, B. Probst, C. Bachmann, R. Alberto, K. K. Baldridge and P. Hamm, Mechanism of Photocatalytic Hydrogen Generation by a Polypyridyl-Based Cobalt Catalyst in Aqueous Solution, Inorg. Chem., 2015, 54, 646-657
[14] D. Lotti, P. Hamm and J. Kraack, Surface-Sensitive Spectro-Electrochemistry Using Ultrafast 2D ATR IR Spectroscopy, J. Phys. Chem. C, 2016, 120, 2883-2892
[15] R. Fernández-Terán and P. Hamm, A Closer Look into the Distance Dependence of Vibrational Energy Transfer on Surfaces Using 2D ATR-IR Spectroscopy, J. Chem. Phys., 2020, 153, 154706
[16] K. Oppelt, M. Mosberger, J. Ruf, R. Fernández-Terán, B. Probst, R. Alberto, P. Hamm, Shedding Light on the Molecular Surface Assembly at the Nanoscale Level: Dynamics of a Re(I) Carbonyl Photosensitizer with a Co-Adsorbed Cobalt Tetrapyridyl Water Reduction Catalyst on Metal Oxides, J. Phys. Chem. C, 2020, 124, 12502–12511