 Fráter Symposium
Fráter SymposiumOrganic Chemistry Institute
From Superambrox to Cubebol:
How Projects Evolve
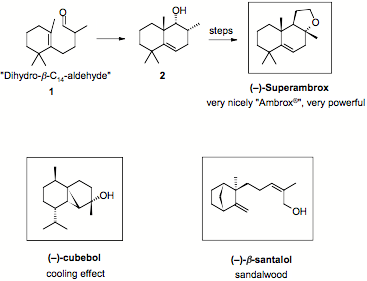
Superambrox represents a highly appreciated, powerful, ambery fragrance compound.
An unprecedented type III ene cyclization of “dihydro-β-C14-aldehyde” 1, a high tonnage commodity, opens a ready access to decalin 2. Its transformation into Superambrox relies on a highly diastereoselective acetoxylation and an original Ir- or Ru-catalyzed OH-assisted isomerization of a 2-buten-1,4-diol.
In the course of the envisaged synthesis of Superambrox, we originally explored the rearrangement of propargylic alcohols into enals. This led us to discover a new copper-catalyzed enynol cycloisomerization, which was studied in more detail.
Taking inspiration from these cycloisomerization reactions, we accomplished a novel synthesis of the sesquiterpene (–)-cubebol, a valuable cooling agent. The key-step consisted of a highly diastereoselective Pt-, Au- or Cu-catalyzed cycloisomerization of an enynol pivalate.
How this project evolved into a new synthesis of the precious sandalwood odorant b-santalol will be discussed in the final part.